Abstract
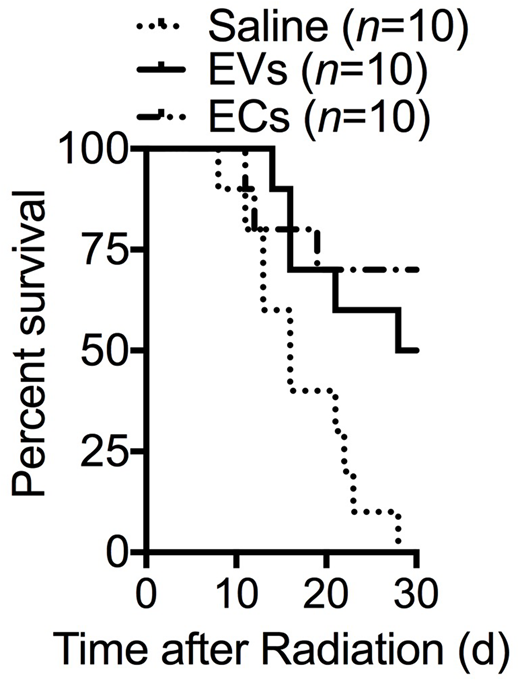
Hematopoietic stem cells (HSCs) reside in specialized microenvironments and adjacent to endothelial cells (ECs), from which they receive regulatory cues. Following injury from radiation or chemotherapy, transplanted ECs can facilitate hematopoietic regeneration. Since these ECs do not engraft in the marrow, we hypothesize that ECs facilitate hematopoietic regeneration through secretion of extracellular vesicles (EVs). EVs are becoming recognized as a mode of cell-to-cell communication through transfer of their cargo of nucleic acids and proteins. Using ultracentrifugation, we visualized these EVs by transmission electron microscopy and measured their size to be a mean size of 160 nm (range 121-217 nm, n=18). Using flow cytometric analysis with Syto13, we demonstrate that these EVs contain nucleic acids in 30% of the particles. Quantitative real-time PCR show that these EVs express CD31 and VECadherin, and not Fibroblast Specific Marker 1 (FSP1), indicating EVs bear EC markers. At day 7 following 300 cGy, C57BL/6 cKit+Sca-1+Lineage- (KSL) cells cultured with EVs demonstrated total cell expansion and colony-forming unit cells (CFCs) compared to cultures with cytokines alone (*p< 0.0001 and 0.01, respectively, n= 3-6/group). Following myelosuppressive, sub-lethal radiation dose of 500 cGy, C57BL/6 mice treated with EVs on days 1-4 display increased marrow cellularity (*p= 0.003, n= 2-3/group), preserved vascular endothelial cells by mouse endothelial cell antigen immunohistochemistry (*p=0.03, n=2-4/group), CD150+CD48-KSL cells (*p= 0.03, n= 5/group), and increased CFCs (*p= 0.03, n= 6/group) compared to saline-treated mice. No differences were detected in these parameters when EV-treated mice were compared to mice treated with primary, cultured C57BL/6 marrow ECs. Following lethal-dose irradiation of 800 cGy, mice that received EVs on day 1-4 display prolonged survival compared to saline-treated mice (*p= 0.008 by Log-rank Analysis, n= 10/group). At day 30 following irradiation, 5 of 10 mice were alive in the EV-treated group compared to 0 of 10 in the saline-treated group. No differences in survival were observed between EV-treated and EC-treated mice (p= 0.4). Treatment of irradiated C57BL/6 hematopoietic stem/progenitor cells (HSPCs) with EVs generated from a genetically distinct strain (BALB/c mice) showed similar levels of cell expansion and CFCs compared to treatment of HSPCs from syngeneic EVs. Our findings show that syngeneic or allogenic EVs could be cell-derived therapy to deliver physiologic doses of nucleic acids and growth factors to hematopoietic cells to accelerate hematopoietic regeneration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.